Single cell isolation and analysis
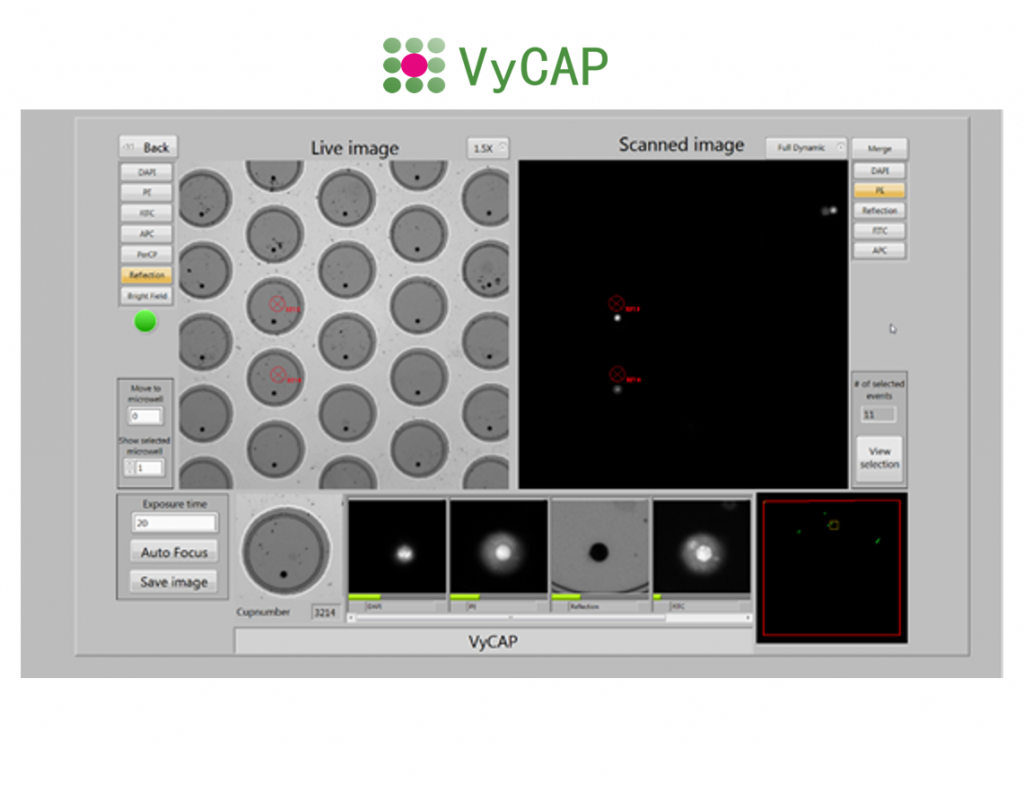
The Puncher system enables single cell imaging, isolation and analysis. The system is based on a Nikon Ti2-E inverted fluorescence microscope that is equipped with dedicated hardware and software to isolate single cells. After the individual cells are isolated they can be genetically analyzed.
Fully automated
The Puncher system is fully automated. It comprises high end scanning stages to quickly position the chip, wells plate and Puncher needle. The additional Puncher needle measuring unit measures the position of the Puncher needle in three dimensions to ensure matching of the needle location with the the microwell chip and cell to be isolated.
Easy loading
Our microwell chip and Puncher needle as well as the 96, 384 wells plates or 0.2ml PCR tubes can be easily loaded on the Puncher system. After loading, the system quickly callibrates the location of the different components.
High end optical components
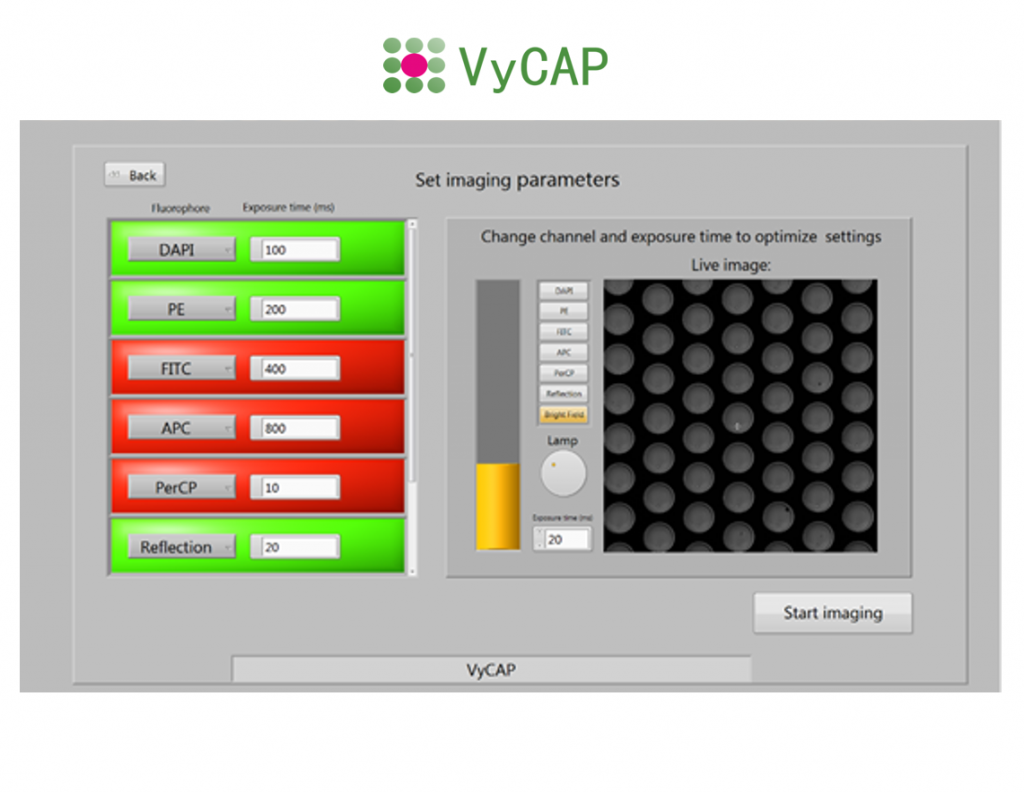
The system uses a Lumencor LED excitation light source and Hamamatsu CMOS scientific camera. This enables the user to acquire high quality fluorescence images of the single cells using a 10x, 20x or 40x objective. Based on the selection criteria the single cells of interest are presented in an automatically generated gallery.
Isolate single cells to a tube or plate
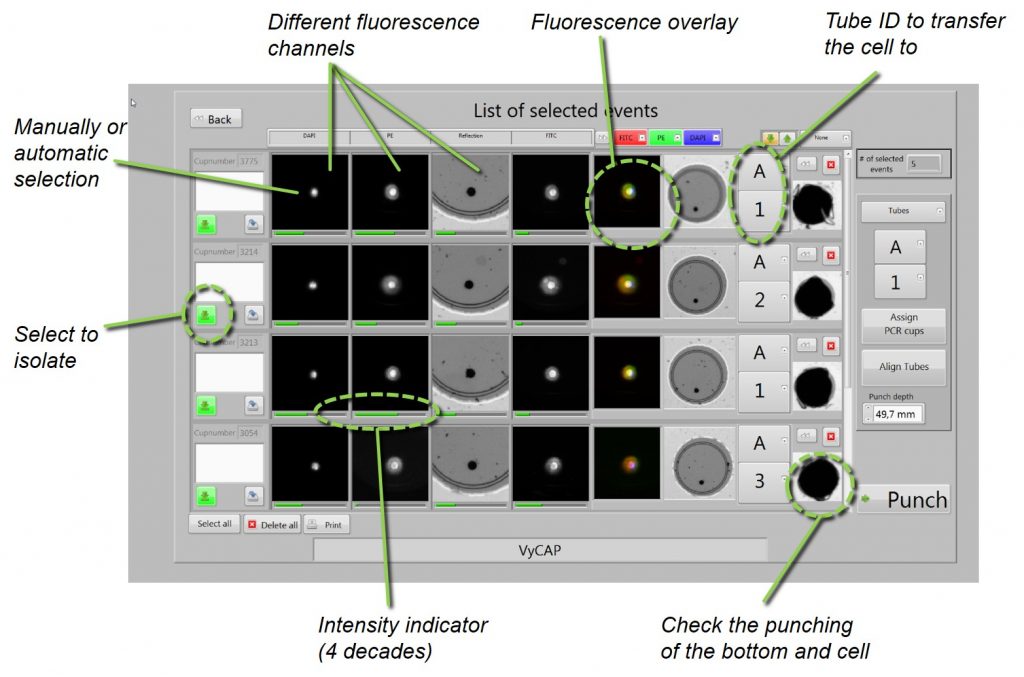
After selecting the cells of interest for genetic single cell analysis. The cells can be isolated into PCR tubes or 96 or 384 plates. Cells are isolated at a rate of 1 cell / second. The punched microwell bottom with the cell drops into a plate or tube. Images of the punched microwells are acquired before and after punching to ensure that the cell is transferred into the tube or well plate.
Report generation
The Puncher system software generates a report after cell isolation. This report contains the cell image gallery and tube number or well plate location into which the cell is transferred. After the single cell analysis this report can be linked to the RNA or DNA data.
Fluorescence imaging
In addition to the single cell isolation, the system can be used as a normal inverted microscope to scan and acquire fluorescence images of the cells collected on our microsieve chip. The microsieve chip is typically used for CTC enumeration.
| Base: | Inverted Nikon TiE-2 microscope |
| Objectives: | 10x NA 0.30, 20x NA 0.45, 40X NA 0.65 |
| Filters cubes: | DAPI, FITC, PE, APC, Reflection |
| LED: | Sola Lumencor Light source |
| Camera: | Hamamatsu CCD camera, Orca Flash 4.0 Hamamatsu CCD camera, Orca Flash 4.0 LT, type C11440-42 |
| Data management: | VyCAP imaging and puncher system software |
| Application: | Enumeration and single cell isolation |
| Imaging time per slide: | Typically 10-20 min |
| Transfer: | 1 cell/second |
| Collection tubes: | 0.2ml Eppendorf tube, 96 or 384 well plates |