VyCAP’s solution for cell line generation uses the unique features of our microwell chip. Viable single cells can be selected and isolated based on their fluorescence, morphology and secreted products. The solution consists of innovative hardware and software in combination with validated protocols.
The cell line generation products comprise three essential parts:
- A Single cell disposable, to distribute single cells in the wells of the microwell chip
- A Clamp unit, to press the microwell chip to the capturing surface with a predefined force
- A Puncher system placed in a flowhood, to automatically select and isolate cells
Unique Selling Points for cell line generation
- Short workflow (<1hr) to select cells for clonal expansion
- Minimal amount of stress on viable cells
- 100% clonality
- High resolution fluorescence images
- Single cell isolation based on the secretome and secreted products
- Validated protocols
Typical workflow for cell line generation
Selection and isolation of single cells for clonal expansion is performed under sterile conditions inside a flow hood. Depending on the cell selection criteria such as cell fluorescence, cell morphology or single cell secretion there are different workflows.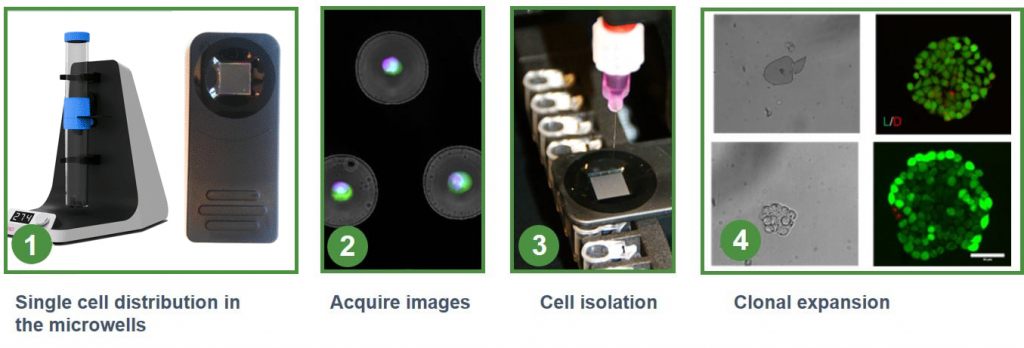
Single cells in 6400 wells
Our microwell chip contains 6400 wells. The bottom of these wells is optically transparent, has a thickness of 1 µm and contains a single pore. A cell suspension is transferred to the microwells chip: fluidic forces direct the cell towards to bottom of the microwells, where the fluid exits through the pore by applying a negative force. This flow stops once a cell is on top of the pore, causing the other cells to flow towards a neighboring well. Filling of the 6400 microwells takes a few minutes and the cells remain inside the preferred cell medium all time. After completing the filling of the microwells, over 99% of the filled microwells contains a single cell.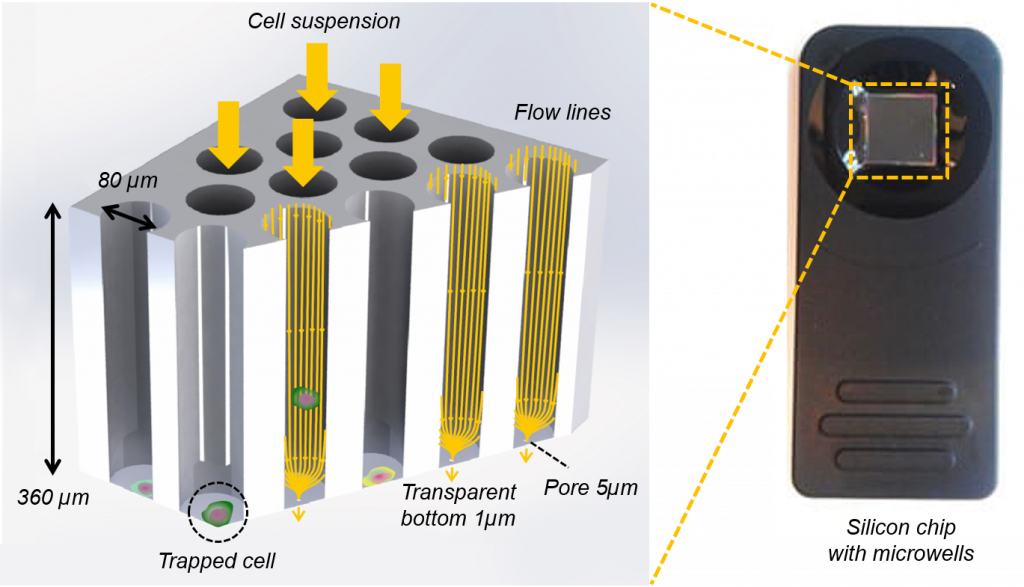
Minimal amount of stress on viable cells
Below an example of MCF-7 cells that are labelled with a live/death staining. Calcein AM staining indicated that over 90% of the cells are viable after filling of the microwells (panel A). The user can isolate the single cells immediately after filling of te wells or place the microwell chip inside an incubator and culture the cells while these remain in the microwells (panel B).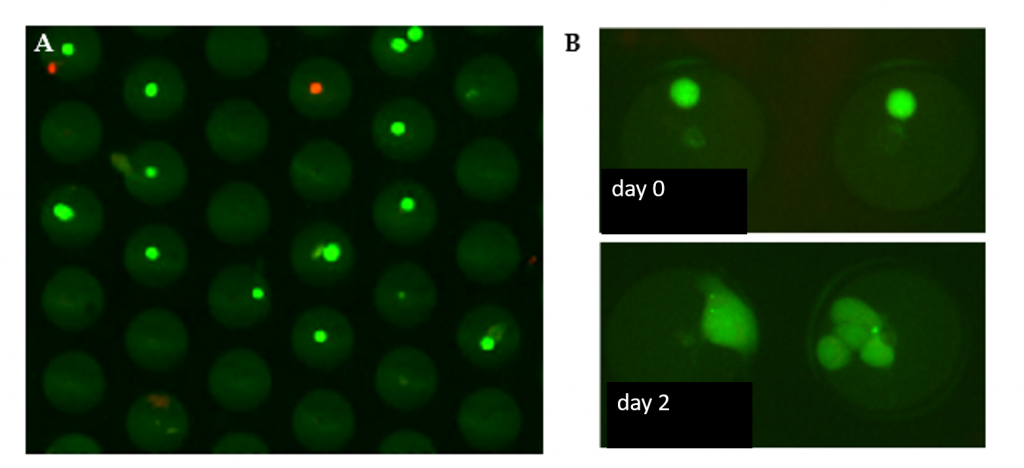
Isolate single cells for clonal expansion
After filling of the microwells the Puncher system acquires images of all cells. Based on their fluorescence signature and other morphology parameters a gallery of cells is presented to the user. The cells that need to be isolated are selected and the Puncher system isolates these fully automatically. During the whole process the cells remain in culture medium. To minimize the cell stress the workflow is designed most efficiently with low pressures and minimal handling steps.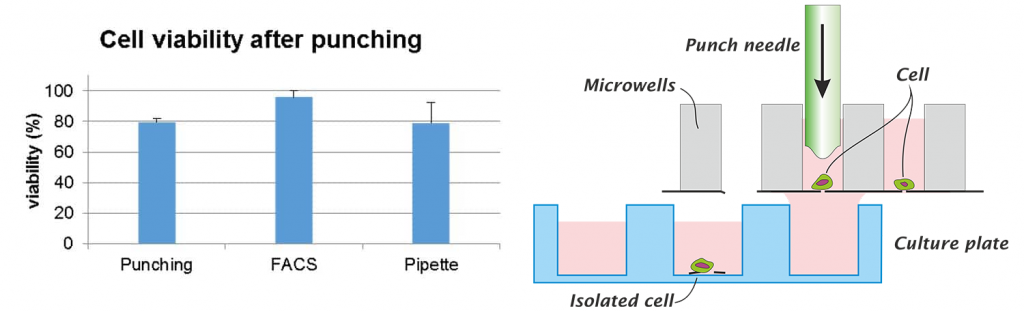
The images below present an example of a single MCF-7 cell that is isolated and next expanded. The Puncher platform has been used to isolate and culture organoids, CTCs, hybridoma and CHO cells.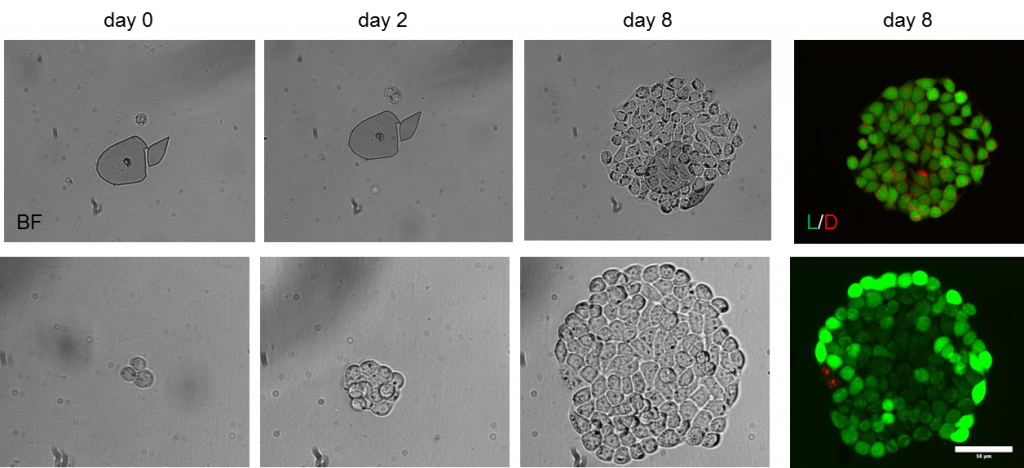
Cell proliferation inside the microwells
Alternatively the microwell chip with single cells can be placed in an incubator to culture the cells inside the microwells. The image below presents the proliferating of a single MCF-7 cell over the course of 9 days. The microwell chips can be coated with a thin layer of proteins i.e. matrigel or fibronection to enhance cell adhesion and proliferation properties of the cells. Work has been performed in close collaboration with the University of Twente.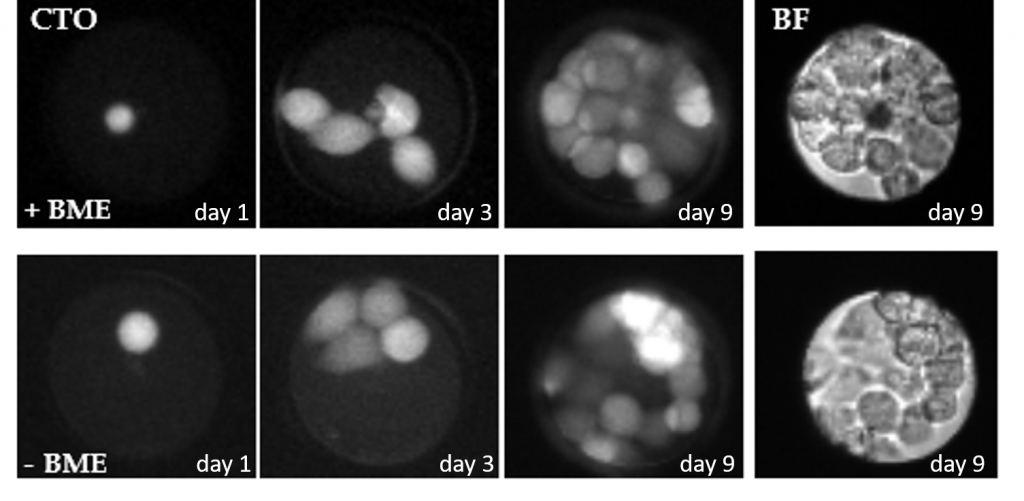
Secretion analysis of 6400 single cells at once
To determine and analyze cell secreted molecules like e.g. cytokines and antibodies, a capturing surface for example a PVDF-membrane is mounted against the bottom of the microwells. The secreted molecules are “printed” by diffusion through the pore in the microwell bottom on the capturing surface. The secreted molecules can be analyzed by common method like ELISA. The location of the captured molecules is correlated with the well number in which the cell that produced these molecules resides. Next this cell can be isolated for clonal expansion. For more details see Applications, Cell line generation.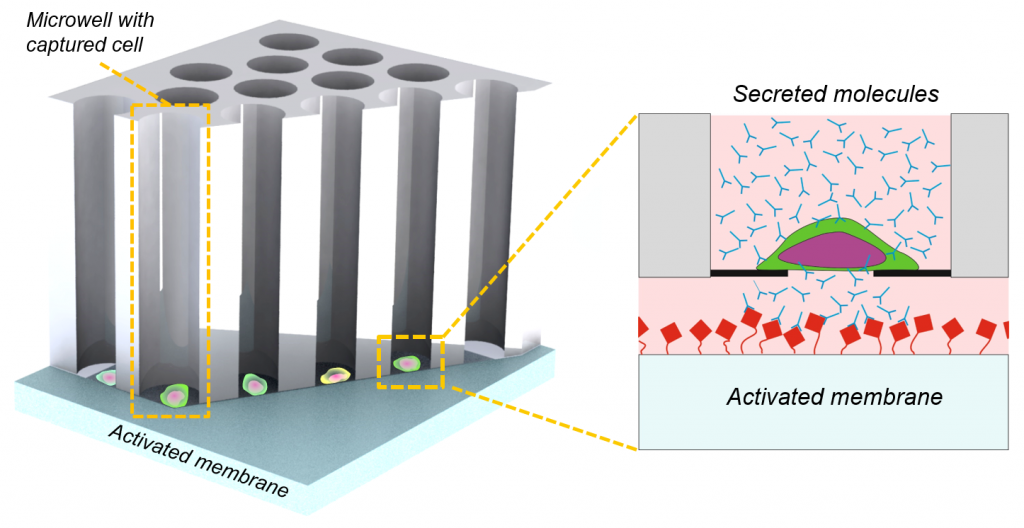
Puncher system inside a flowhood
To maintain sterile conditions the Puncher system is placed in a flowhood. VyCAP has designed a dedicated flowhood with integrated monitor and cable connections from the Puncher microscope to the electronics below the flow cabinet.

